Enhanced TDS
Identification & Functionality
- Chemical Name
- Ingredient Origin
- Supplied By
- Pharma & Nutraceuticals Functions
- CAS No.
- 9004-65-3
- EC No
- 618-389-6
- Technologies
- Product Families
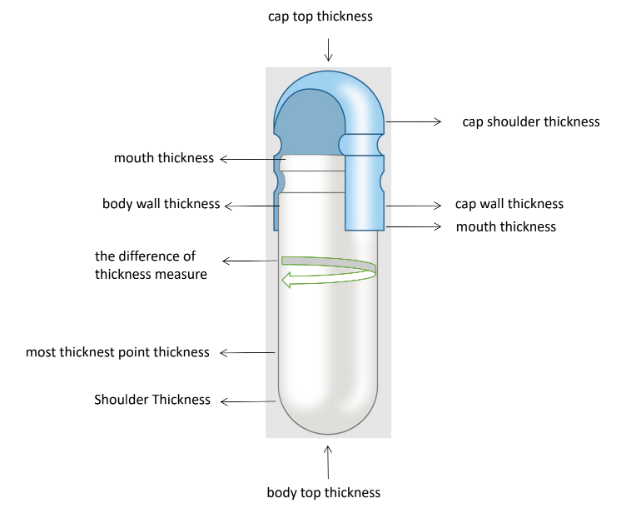
- Capsule Diagram
Features & Benefits
- Labeling Claims
- Key Features
- High quality
- Excellent machinability on both high-speed and semi-automatic filling machines.
- Derived from 100% vegetable material.
- No cross-linking reaction.
- Complies with USP / EP / ChP regulations.
- Suitable for hygroscopic and moisture sensitive ingredients.
Applications & Uses
- Markets
- Applications
- Segments
- Applications
- Dosage Form
Properties
- Physical Form
Regulatory & Compliance
Packaging & Availability
- Packaging Type
- Regional Availability
- Packaging Information
Carton boxes with an 10 fold corrugated fiber board liner and two inner PE bags to protect the capsules from heat sources and other environmental extremes.
Carton Count:
Size Quantity 1 140,000
Storage & Handling
- Storage Conditions
- Validity date is supported if recommendations for storage are observed (recommended:10 ~ 30°C and 35% ~ 65% relative humidity)
- Cartons should be stored on pallets (right side up) in a well protected area.
- Heat sources such as space heaters or sunlight through windows should be avoided.
- Capsules should be kept more than one meter away from steam pipes or incandescent electric lamp.
- Capsules should be kept more than 15 cm away from the wall of the building.
